Journal of the Faculty of Engineering and Architecture of Gazi University 33:2 (2018) 459-472
Meltem Doğan , Saliha Çetinyokuş Kılıçarslan, Emre Kırcı
Abstract
In the study, it was aimed to prepare chromium-based catalysts with high activity and selectivity for isobutane dehydrogenation. Monochromate amount in the structure of chromium-based catalysts was identified as the main factor affecting the activity and selectivity. The commercial activated carbon (AC) support showed activity for propene formation reaction as well as isobutane dehydrogenation. First part of the experimental studies were carried out at four different contact times of metal salt solution and support namely, 4, 24, 48, 72 hours of mixing times. The highest amount of monochromate was observed in the structure of the catalyst prepared at mixing time of 24 hours. The highest selectivity value in the catalytic tests was also obtained with the catalyst prepared at the same mixing time. When the amount of metal in the catalyst structure was increased from 3% to 6% (by mass), the amount of monochromate increased but, the amount of monochromate decreased at concentrations higher than 6%. It was found that the chromium remained mostly in inactive Cr2O3 crystals, when the amount of chromium was increased to 22%. It was predicted that selectivity reduced because of the occurrence of side reactions on the catalysts containing high amount of chromium. In this study the effect of Ca addition on the structure and activity of catalyst was also investigated. As the Ca/Cr ratio was increased, the amount of monochromate decreased. Very low isobutane conversion and isobutene selectivity values were observed in the studies carried out with calcium containing catalysts. The amount of monochromate formed increased when the calcination was performed in CO2 medium and at high temperatures. Our study, which showed how the synthesis conditions affect the amount of monochromate, is thought to provide important contributions to the literature on chromium-based catalyst preparation studies
Purpose
It was aimed to prepare chromium-based catalysts with high activity and selectivity for isobutane dehydrogenation. Monochromate amount in the structure of chromium-based catalysts was identified as the main factor affecting the activity and selectivity. For this reason, it was attempted to determine the catalyst preparation conditions required to obtain high amount of monochromate form.
Theory and Methods
The impregnation method was used in catalyst preparation studies. The effects of mixing time, chromium content, calcium addition and calcination conditions on the structure and activity of the prepared catalysts were investigated. Physisorption, XPS, DR-UV-vis, XRD analyzes were used in the characterization studies of the synthesized catalysts. The catalysts were placed in a quartz tube and catalytic tests were carried out under fixed bed reactor conditions. The isobutane dehydrogenation reaction was carried out at 600°C and at atmospheric pressure for two hours. Samples from the reactor outlet were analyzed by gas chromatography.
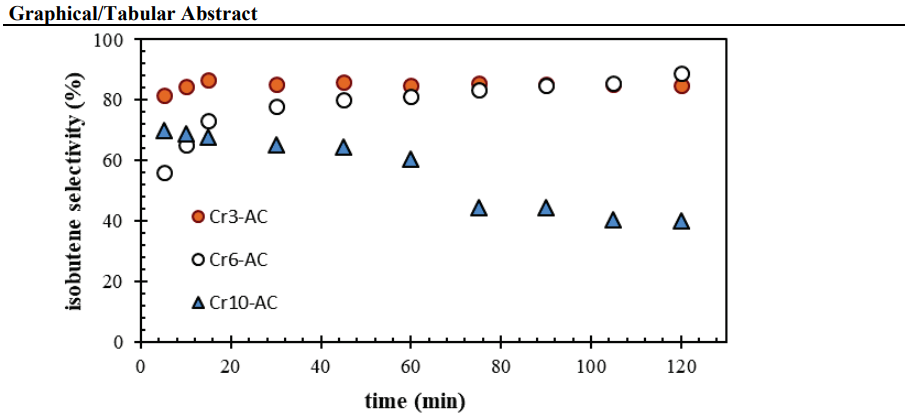
Results: It was determined that AC support itself had activity for propene formation reaction as well as isobutane dehydrogenation. The highest amount of monochromate was observed in the structure of the catalyst prepared at mixing time of 24 hours. It was found that the chromium remained mostly in inactive Cr2O3 crystals, when the amount of chromium was increased to 22%. It was predicted that selectivity reduced because of the occurrence of side reactions on the catalysts containing high amount of chromium. As the Ca/Cr ratio was increased, the amount of monochromate decreased. The amount of monochromate formed increased when the calcination was performed in CO2 medium and at high temperatures.
Conclusion
Monochromate structures were observed in all of the synthesized catalysts. Higher isobutene selectivity values were obtained for catalysts with high amount of monochromate in the structure. At low mixing times, very low amount of monochromate has been determined. When the amount of metal increased above 6%, there was a decrease in monochromate amounts, resulting in a decrease in isobutene selectivity. By covering the active centers of the added calcium, very low isobutane conversion values were obtained in the catalysts. It has been shown that the calcination temperature must be high in order to have a high amount of monochromate in the structure.
Referances
- Sattler J.J.H.B., Ruiz-Martinez J., Santillan-Jimenez E., Weckhuysen B.M., Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides, Chem. Rev., 114, 10613−10653, 2014.
- Rashidi M., Nikazar M., Rahmani M., Mohamadghasemi, Z., Kinetic modeling of simultaneous dehydrogenation of propane and isobutane on Pt-Sn-K/Al2O3 catalyst, Chemical Engineering Research and Design, 95, 239–247, 2015.
- Fang D., Zhao J., Liu S., Zhang L., Ren W., Zhang H., Relationship between Cr-Al Interaction and the Performance of Cr-Al2O3 Catalysts for Isobutane Dehydrogenation, Modern Research in Catalysis, 4, 50-58, 2015.
- Kilicarslan S., Dogan M., Dogu T., Cr Incorporated MCM-41 type catalysts for isobutane dehydrogenation and deactivation mechanism, Ind. Eng. Chem. Res. 52, 3674–3682, 2013.
- Cetinyokus Kilicarslan S., Dogan M., Dogu T., Contribution of Pd Membrane to Dehydrogenation of Isobutane Over a New Mesoporous Cr/MCM-41, Int. J. Chem. React. Eng., 14, 3, 727–736, 2016.
- Zhang D., Li X., Qin B., Li X., Guo X., Lai C., Fabrication of Chromium (III) Oxide (Cr2O3) Coating by Electrophoretic Deposition, Journal of The American Ceramic Society, 97, 11, 3413-3417, 2014.
- Wang L., Zhang Y., Lou Y., Guo Y., Lu G., Guo Y., Pd catalyst supported on activated carbon honeycomb monolith for CO oxidation and the application in air purification of vehicular tunnel, Fuel Processing Technology, 122, 23–29, 2014.
- Trawczynski J., Gheek P., Okal J., Zawadzki M., Ilan Gomez M.J., Reduction of nitrate on active carbon supported Pd-Cu catalysts, Applied Catalysis A: General, 409– 410, 39– 47, 2011.
- Fan Q.Y., Xiu G.J., Hao C.Y., Chao S.M., Qiang Y.H., The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts, Applied Surface Science, 282, 425– 431, 2013.
- Ding J.F., Qin Z.F., Li X.K., Wang G.F., Wang J.G., Coupling dehydrogenation of isobutane in the presence of carbon dioxide over chromium oxide supported on active carbon, Chinese Chemical Letters 19, 1059–1062, 2008.
- Ding J., Qin Z., Li X., Wang G., Wang J., Catalytic dehydrogenation of isobutane in the presence of carbon dioxide over nickel supported on active carbon, Journal of Molecular Catalysis A: Chemical, 315, 221–225, 2010.
- Hong Y., Liwu L., Qingxi W., Longy X.U., Sujuan X.I.E., Shenglin L.I.U., Oxidative dehydrogenation of ethane with carbon dioxide to ethylene over Cr-loaded active carbon catalyst, Studies in Surface Science and Catalysis, 87-92, 2001.
- Wegrzyniak A., Jarczewski S., Wach A., Hedrzak E., Ku´strowski P., Michorczyk P., Catalytic behaviour of chromium oxide supported on CMK-3 carbonreplica in the dehydrogenation propane to propene, Applied Catalysis A: General, 508, 1–9, 2015.
- Diaz V.J.J., Sua´rez L.M.C., Figueiredo J.L., Oxidative dehydrogenation of isobutane over activated carbon catalysts, Applied Catalysis A: General, 311, 51–57, 2006.
- Gniot I., Kirszensztejn P., Kozłowski M., Oxidative dehydrogenation of isobutane using modified activated carbons as catalysts, Applied Catalysis A: General, 362, 67–74, 2009.
- Xu L., Liu Y., Li Y., Lin Z., Ma X., Zhang Y., Argyle M.D., Fan M., Catalytic CH4 reforming with CO2 over activated carbon based catalysts, Applied Catalysis A: General, 469, 387– 397, 2014.
- Shee, D., Sayri, A., Light Alkane Dehydrogenation Over Mesoporous Cr2O3/Al2O3, Catalysts. Appl. Catal. A, 389, 155, 2010.
- Mahendinan C., Sangeetha, P., Vijayan, P., Sardhar Basha, S. J., Shanthi, K., Vapor Phase Oxidation of Tatralin over Cr and Fe Substituted MCM-41 Molecular Sieves, J. Mol. Catal A: Chem., 275, 84, 2007.
- Zhang L., Zhao Y., Dai H., He H., Au C. T., Comparative Investigation on The Properties of Cr-SBA-15 and CrOx/SBA-15, Catalyis Today, 131, 42, 2008.
- Maldonado F., Rivera R., Stashans A., Structure, electronic and magnetic properties of Ca-doped chromium oxide studied by the DFT method, Physica B, 407, 1262–1267, 2012.
- Neri G., Pistone A., De Rossi S., Rombi E., Milone C., Galvagno S., Ca-doped chromium oxide catalysts supported on alümina for the oxidative dehydrogenation of isobutane, Applied Catalysis A: General, 260, 75–86, 2004.
- Cetinyokus Kilicarslan S., Dogan M., Dogu T., Synhthesis and Characterization of Ca-Cr-MCM-41 Catalysts for Isobutane Dehydrogenation, Journal of the Faculty of Engineering and Architecture of Gazi University, 29, 3, 459-467, 2014.

